Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy
Abstract
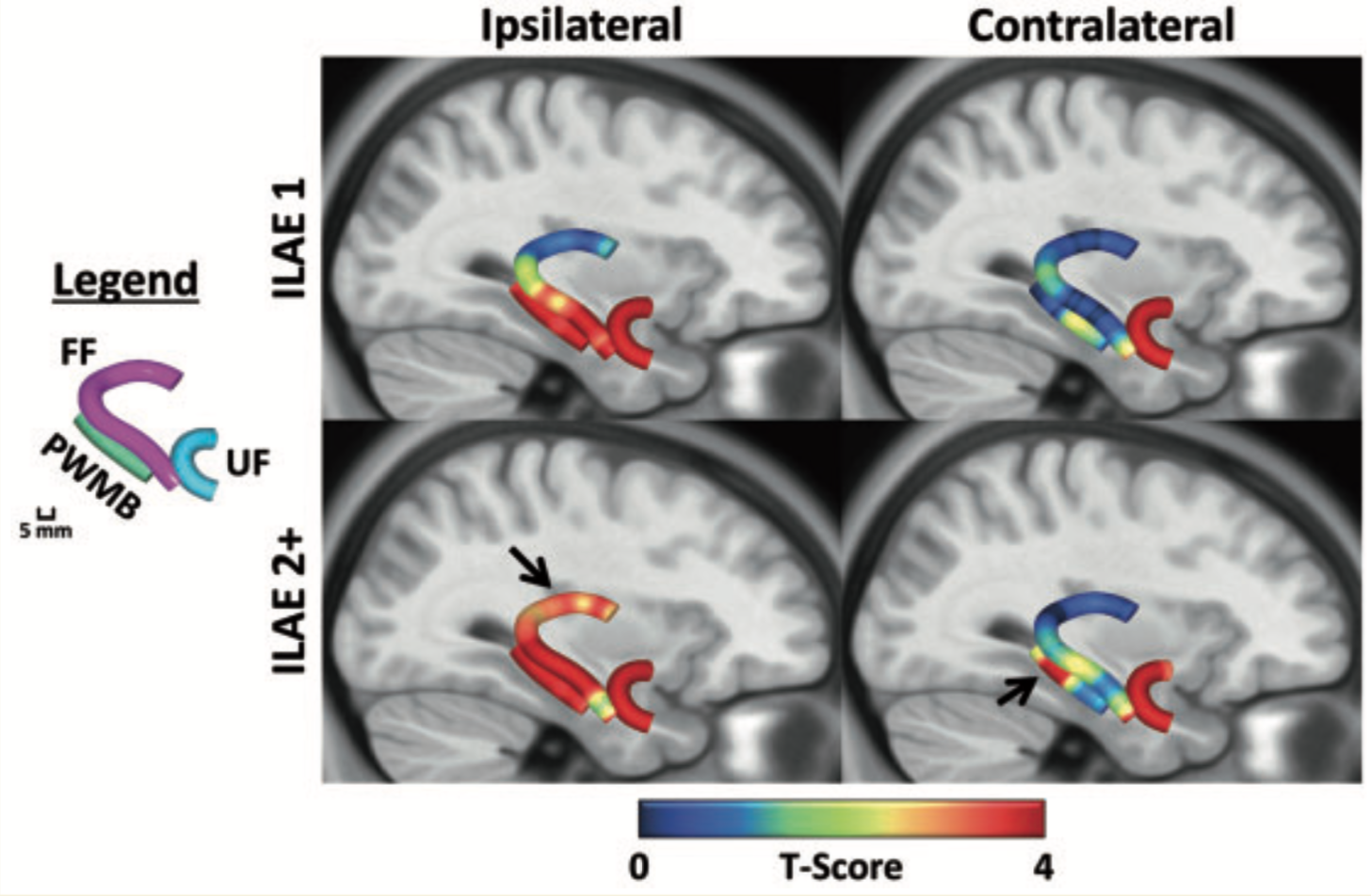
Approximately one in every two patients with pharmacoresistant temporal lobe epilepsy will not be rendered completely seizure-free after temporal lobe surgery. The reasons for this are unknown and are likely to be multifactorial. Quantitative volumetric magnetic resonance imaging techniques have provided limited insight into the causes of persistent postoperative seizures in patients with temporal lobe epilepsy. The relationship between postoperative outcome and preoperative pathology of white matter tracts, which constitute crucial components of epileptogenic networks, is unknown. We investigated regional tissue characteristics of preoperative temporal lobe white matter tracts known to be important in the generation and propagation of temporal lobe seizures in temporal lobe epilepsy, using diffusion tensor imaging and automated fibre quantification. We studied 43 patients with mesial temporal lobe epilepsy associated with hippocampal sclerosis and 44 healthy controls. Patients underwent preoperative imaging, amygdalohippocampectomy and postoperative assessment using the International League Against Epilepsy seizure outcome scale. From preoperative imaging, the fimbria-fornix, parahippocampal white matter bundle and uncinate fasciculus were reconstructed, and scalar diffusion metrics were calculated along the length of each tract. Altogether, 51.2% of patients were rendered completely seizure-free and 48.8% continued to experience postoperative seizure symptoms. Relative to controls, both patient groups exhibited strong and significant diffusion abnormalities along the length of the uncinate bilaterally, the ipsilateral parahippocampal white matter bundle, and the ipsilateral fimbria-fornix in regions located within the medial temporal lobe. However, only patients with persistent postoperative seizures showed evidence of significant pathology of tract sections located in the ipsilateral dorsal fornix and in the contralateral parahippocampal white matter bundle. Using receiver operating characteristic curves, diffusion characteristics of these regions could classify individual patients according to outcome with 84% sensitivity and 89% specificity. Pathological changes in the dorsal fornix were beyond the margins of resection, and contralateral parahippocampal changes may suggest a bitemporal disorder in some patients. Furthermore, diffusion characteristics of the ipsilateral uncinate could classify patients from controls with a sensitivity of 98%; importantly, by co-registering the preoperative fibre maps to postoperative surgical lacuna maps, we observed that the extent of uncinate resection was significantly greater in patients who were rendered seizure-free, suggesting that a smaller resection of the uncinate may represent insufficient disconnection of an anterior temporal epileptogenic network. These results may have the potential to be developed into imaging prognostic markers of postoperative outcome and provide new insights for why some patients with temporal lobe epilepsy continue to experience postoperative seizures.